
Q. Consider the following two parallel irreversible first order reactions at temperature T,
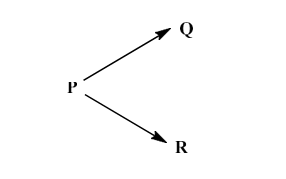
where 𝑘1and 𝑘2 are the rate constants and their values are 5 × 10–2 and 15 × 10–2 min–1, respectively, at temperature T. If the initial concentration of the reactant ‘P’ is 4 mol L–1, then the concentration of product ‘R’ after 10 min of reaction is __________mol L–1. (Round off to two decimal places)
(Assume only P is present at the beginning of the reaction.)
Ans: 2.50 – 2.65
Solution:
First Order Reaction is a chemical reaction in which the rate of the reaction is directly proportional to the concentration of one reactant.
R → P, ln [A] = -k t + ln [A0]
Calculation:-
[R]t = k2 / k1 + k2 [P]o (1 – e-1(k1+k2)t) ⇒
[R]t = 15 x 10-2 min-1 / (5+15) x 10-2 min-1 [4 mol L-1] (1 – e-1(5+15)x 10-2 x10) ⇒
[R]t = 3 (1 – e-20 x 1/100 x 10) ⇒
[R]t = 2.59 mol L-1



![Determine the correctness (or otherwise) of the following Assertion [A] and the Reason [R]](https://www.gkseries.com/blog/wp-content/uploads/2023/10/Determine-the-correctness-or-otherwise-of-the-following-Assertion-A-and-the-Reason-R.jpg)
