
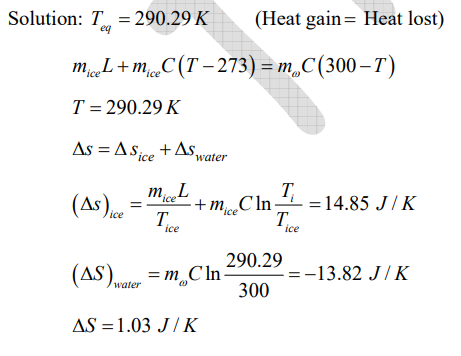
Q. In a thermally insulated container, 0.01 kg of ice at 273 K is mixed with 0.1 kg of water at 300 K. Neglecting the specific heat of the container, the change in the entropy of the system in J/K on attaining thermal equilibrium (rounded off to two decimal places) is (Specific heat of water is 4.2 kJ/kg-K and the latent heat of ice is 335 kJ/kg).
Ans: 1.03
Sol:



![Determine the correctness (or otherwise) of the following Assertion [A] and the Reason [R]](https://www.gkseries.com/blog/wp-content/uploads/2023/10/Determine-the-correctness-or-otherwise-of-the-following-Assertion-A-and-the-Reason-R.jpg)
