
Q. One kilogram of air is compressed at constant temperature of 150 ˚C until its volume is halved. Considering gas constant R = 0.287 kJ/kg-K for air, magnitude of heat rejected (in kJ) in the compression process is
Ans: 84 – 84.3
Sol:
First Law of Thermodynamics: δQ = dU + δW
For Isothermal process dU = 0
∴ δQ = δW
For Isothermal Process:
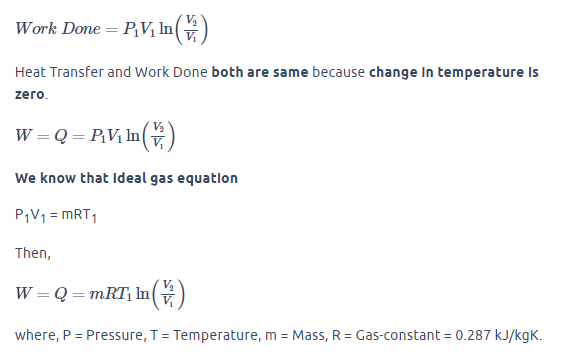
Calculation:
m = 1 kg, T1 = T2 = 150°C = 423 K (Isothermal Process), V1 = 2V2, R = 0.287 kJ/kgK
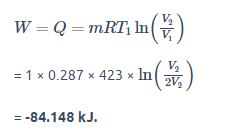
Here, the negative sign shows that the heat is rejected from the system. So, Q = 84.148 kJ



![Determine the correctness (or otherwise) of the following Assertion [A] and the Reason [R]](https://www.gkseries.com/blog/wp-content/uploads/2023/10/Determine-the-correctness-or-otherwise-of-the-following-Assertion-A-and-the-Reason-R.jpg)
