Rapid Response Regulatory Mechanism for COVID-19 pandemic
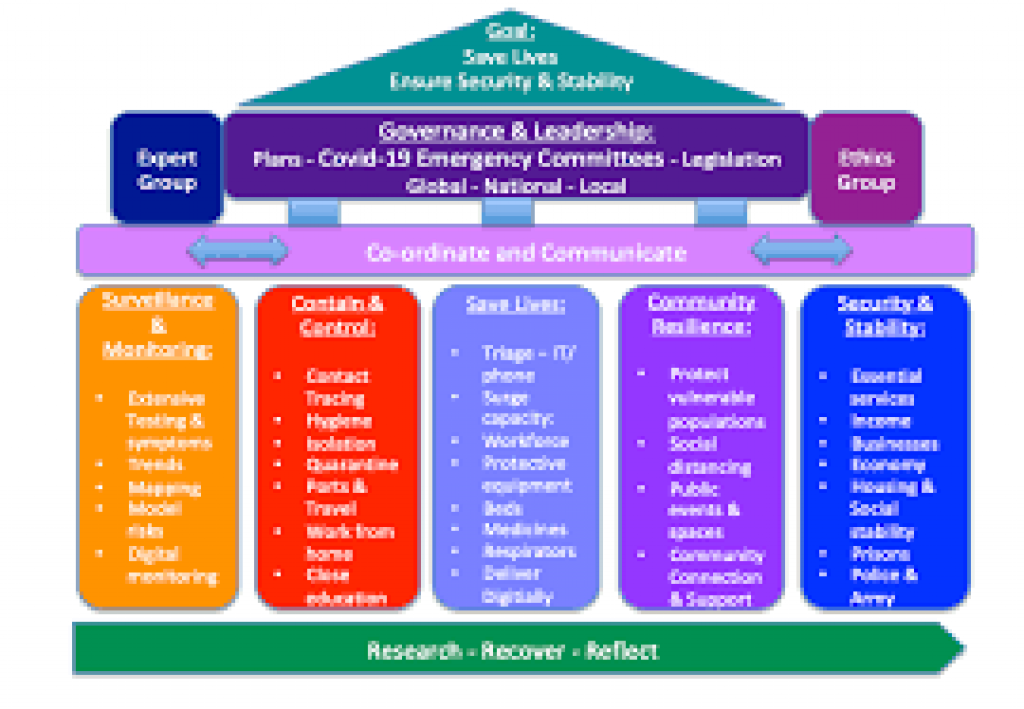
The Department of Biotechnology (DBT) has given out information on the various measures taken to streamline the biosafety regulation and to facilitate researchers and industries which are undertaking research and development in recombinant DNA technology and hazardous microorganisms.
DBT has operationalized the Indian Biosafety Knowledge Portal.
The Department has issued the Revised Guidelines in January 2020. In the guidelines, the Institutional Biosafety Committee has been delegated authority to make decisions on applications of import-export and exchange of GE organisms and products thereof for R&D purposes for RG1 and RG2 items.
DBT has proactively taken several steps to facilitate researchers and industries involved in research on COVID-19.
Guidelines issued by DBT are:
Rapid Response Regulatory Framework for COVID-19 to deal with an application for the development of vaccines, diagnostics, prophylactics and therapeutic
Interim Guidance Document on Laboratory Biosafety to Handle COVID-19 Specimens
IBSCs can conduct their meeting through video conferencing up to 30 June 2020.
Rapid response regulatory framework for the development of recombinant DNA COVID 19 vaccine.