The outer orbitals of C in ethene molecule can be considered to be hybridized to give three equivalent sp² orbitals. The total number of sigma (s) and pi (p) bonds in ethene molecule is
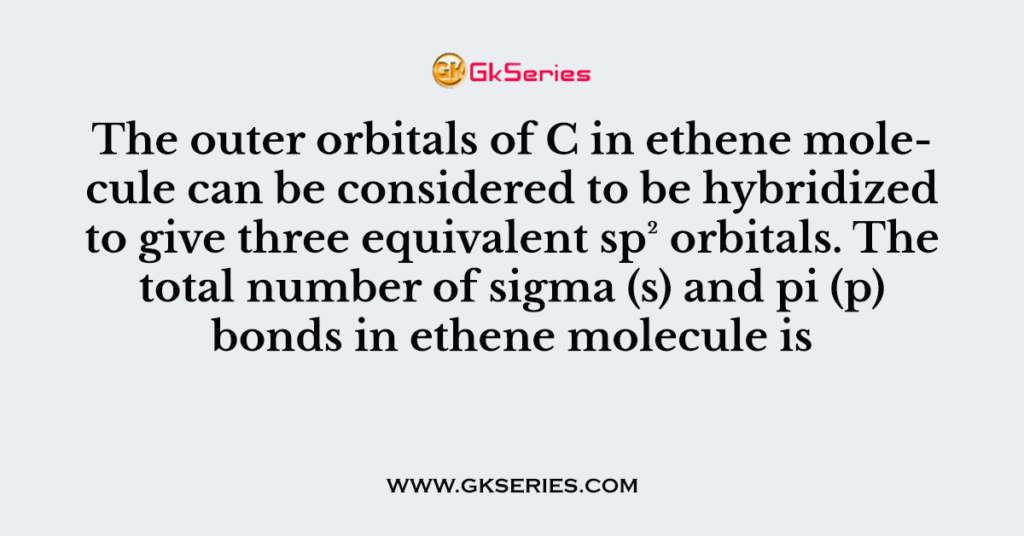
Q. The outer orbitals of C in ethene molecule can be considered to be hybridized to give three equivalent sp² orbitals. The total number of sigma (s) and pi (p) bonds in ethene molecule is
- 1 sigma (s) and 2 pi (p) bonds
- 3 sigma (s) and 2 pi (p) bonds
- 4 sigma (s) and 1 pi (p) bonds
- 5 sigma (s) and 1 pi (p) bonds
Answer: 5 sigma (s) and 1 pi (p) bonds