Two different beakers contain M1-O-H, and M2-O-H solutions separately. Find the nature of the two solutions if the electronegativity of M1 = 3.4, M2 = 1.2, O = 3.5, H = 2.1
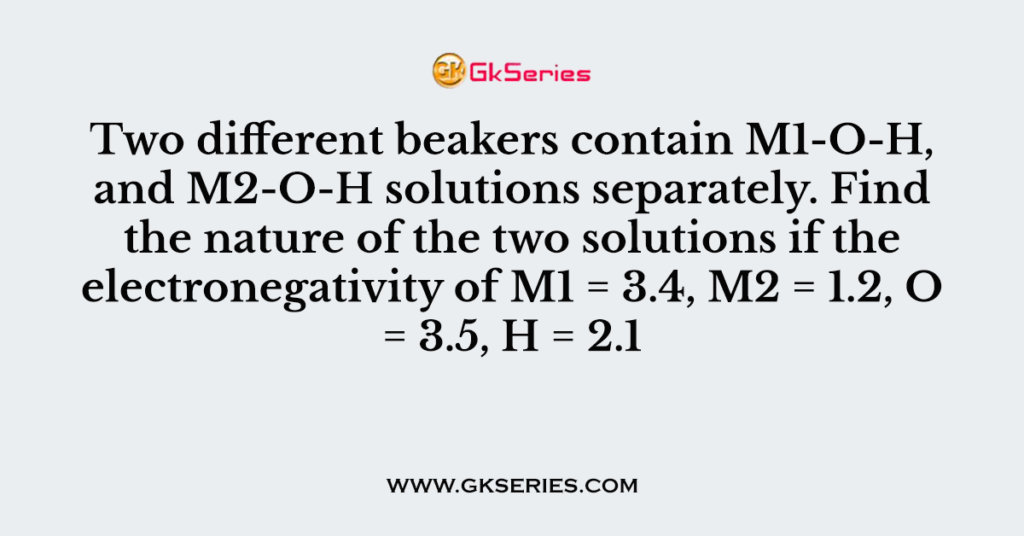
Q. Two different beakers contain M1-O-H, and M2-O-H solutions separately. Find the nature of the two solutions if the electronegativity of M1 = 3.4, M2 = 1.2, O = 3.5, H = 2.1
A. acidic, acidic
B. basic, acidic
C. basic, basic
D. acidic, basic
Answer: acidic, basic