Aldehyde, any of a class of organic compounds in which a carbon atom shares a double bond with an oxygen atom, a single bond with a hydrogen atom, and a single bond with another atom or group of atoms (designated R in general chemical formulas and structure diagrams).
Both aldehydes and ketones contain a carbonyl group, a functional group with a carbon-oxygen double bond. The names for aldehyde and ketone compounds are derived using similar nomenclature rules as for alkanes and alcohols, and include the class-identifying suffixes –al and –one, respectively.
Nomenclature and Structure of Carbonyl Group
Nomenclature
- Aldehydes and ketones: Aldehydes and ketones are the simplest and most important carbonyl compounds. There are two systems of nomenclature of aldehydes and ketones.
- Common names: Aldehydes and ketones are often called by their common names instead of IUPAC names. The common names of most aldehydes are derived from the common names of the corresponding carboxylic acids by replacing the ending –ic of acid with aldehyde.
- IUPAC names: The IUPAC names of open chain aliphatic aldehydes and ketones are derived from the names of the corresponding alkanes by replacing the ending –e with –al and –one respectively.
Structure of the Carbonyl Group: The carbonyl carbon atom is sp2-hybridised and forms three sigma (σ) bonds. The fourth valence electron of carbon remains in its p-orbital and forms a π-bond with oxygen by overlap with p-orbital of an oxygen.
Preparation of Aldehydes and Ketones
Some important methods for the preparation of aldehydes and ketones are as follows:
- By oxidation of alcohols: Aldehydes and ketones are generally prepared by oxidation of primary and secondary alcohols, respectively.
- By dehydrogenation of alcohols: This method is suitable for volatile alcohols and is of industrial application. In this method alcohol vapours are passed over heavy metal catalysts (Ag or Cu). Primary and secondary alcohols give aldehydes and ketones, respectively.
- From hydrocarbons:
- By ozonolysis of alkenes: As we know, ozonolysis of alkenes followed by reaction with zinc dust and water gives aldehydes, ketones or a mixture of both depending on the substitution pattern of the alkene.
- By hydration of alkynes: Addition of water to ethyne in the presence of H2SO4 and HgSO4 gives acetaldehyde. All other alkynes give ketones in this reaction.
Preparation of Aldehydes
- From acyl chloride (acid chloride): Acyl chloride (acid chloride) is hydrogenated over catalyst, palladium on barium sulphate. This reaction is called Rosenmund reduction.
- From nitriles and esters: Nitriles are reduced to corresponding imine with stannous chloride in the presence of hydrochloric acid, which on hydrolysis give corresponding aldehyde.
- From hydrocarbons: Aromatic aldehydes (benzaldehyde and its derivatives) are prepared from aromatic hydrocarbons by the following methods:
- By oxidation of methylbenzene: Strong oxidising agents oxidise toluene and its derivatives to benzoic acids.
- By side chain chlorination followed by hydrolysis: Side chain chlorination of toluene gives benzal chloride, which on hydrolysis gives benzaldehyde. This is a commercial method of manufacture of benzaldehyde.
- By Gatterman – Koch reaction: When benzene or its derivative is treated with carbon monoxide and hydrogen chloride in the presence of anhydrous aluminium chloride or cuprous chloride, it gives benzaldehyde or substituted benzaldehyde.
Preparation of Ketones
- From acyl chlorides: Treatment of acyl chlorides with dialkylcadmium, prepared by the reaction of cadmium chloride with Grignard reagent, gives ketones.
- From nitriles: Treating a nitrile with Grignard reagent followed by hydrolysis yields a ketone.
- From benzene or substituted benzenes: When benzene or substituted benzene is treated with acid chloride in the presence of anhydrous aluminium chloride, it affords the corresponding ketone. This reaction is known as Friedel-Crafts acylation reaction.
Physical Properties
Methanal is a gas at room temperature. Ethanal is a volatile liquid. Other aldehydes and ketones are liquid or solid at room temperature.
The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses. It is due to weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.
Also, their boiling points are lower than those of alcohols of similar molecular masses due to absence of intermolecular hydrogen bonding.
The lower members of aldehydes and ketones such as methanal, ethanal and propanone are miscible with water in all proportions, because they form hydrogen bond with water.
Chemical Reactions
- Nucleophilic addition reactions: Contrary to electrophilic addition reactions observed in alkenes the aldehydes and ketones undergo nucleophilic addition reactions.
- Mechanism of nucleophilic addition reactions: A nucleophile attacks the electrophilic carbon atom of the polar carbonyl group from a direction approximately perpendicular to the plane of sp2 hybridised orbitals of carbonyl carbon.
- Reactivity: Aldehydes are generally more reactive than ketones in nucleophilic addition reactions due to steric and electronic reasons.
- Some important examples of nucleophilic addition and nucleophilic addition-elimination reactions: (a) Addition of hydrogen cyanide (HCN), (b) Addition of sodium hydrogensulphite, (c) Addition of Grignard reagents, (d) Addition of alcohols, (e) Addition of ammonia and its derivatives.
- Reduction:
- Reduction to alcohols: Aldehydes and ketones are reduced to primary and secondary alcohols respectively by sodium borohydride (NaBH4) or lithium aluminium hydride (LiAlH4) as well as by catalytic hydrogenation.
- Reduction to hydrocarbons: The carbonyl group of aldehydes and ketones is reduced to CH2 group on treatment with zinc- amalgam and concentrated hydrochloric acid [Clemmensen reduction] or with hydrazine followed by heating with sodium or potassium hydroxide in high boiling solvent such as ethylene glycol (Wolff-Kishner reduction).
- Oxidation:
Aldehydes differ from ketones in their oxidation reactions. Aldehydes are easily oxidised to carboxylic acids on treatment with common oxidising agents like nitric acid, potassium permanganate, potassium dichromate, etc. Even mild oxidising agents, mainly Tollens’ reagent and Fehlings’ reagent also oxidise aldehydes.
- Reactions due to a-hydrogen:
Acidity of α-hydrogens of aldehydes and ketones: The aldehydes and ketones undergo a number of reactions due to the acidic nature of α-hydrogen. The acidity of α-hydrogen atoms of carbonyl compounds is due to the strong electron withdrawing effect of the carbonyl group and resonance stabilisation of the conjugate base.
- Other reactions:
- Cannizzaro reaction: Aldehydes which do not have an α-hydrogen atom, undergo self oxidation and reduction (disproportionation) reaction on heating with concentrated alkali.
- Electrophilic substitution reaction: Aromatic aldehydes and ketones undergo electrophilic substitution at the ring in which the carbonyl group acts as a deactivating and meta-directing group.
Uses of Aldehydes and Ketones
In chemical industry aldehydes and ketones are used as solvents, starting materials and reagents for the synthesis of other products.
Formaldehyde is well known as formalin (40%) solution used to preserve biological specimens and to prepare bakelite (a phenol-formaldehyde resin), urea-formaldehyde glues and other polymeric products.
Acetaldehyde is used primarily as a starting material in the manufacture of acetic acid, ethyl acetate, vinyl acetate, polymers and drugs.
Benzaldehyde is used in perfumery and in dye industries. Acetone and ethyl methyl ketone are common industrial solvents.
Many aldehydes and ketones, e.g., butyraldehyde, vanillin, acetophenone, camphor, etc. are well known for their odours and flavours.
Carboxylic Acids
Carbon compounds containing a carboxyl functional group, –COOH are called carboxylic acids. The carboxyl group, consists of a carbonyl group attached to a hydroxyl group, hence its name carboxyl.
Nomenclature and Structure of Carboxyl Group
The IUPAC system of nomenclature assigns a characteristic suffix to these classes. The –e ending is removed from the name of the parent chain and is replaced -anoic acid. Many carboxylic acids are called by the common names. These names were chosen by chemists to usually describe a source of where the compound is found. In common names of aldehydes, carbon atoms near the carboxyl group are often designated by Greek letters. The atom adjacent to the carbonyl function is alpha, the next removed is beta and so on.
Methods of Preparation of Carboxylic Acids
- From primary alcohols and aldehydes: Primary alcohols are readily oxidised to carboxylic acids with common oxidising agents such as potassium permanganate (KMnO4) in neutral, acidic or alkaline media or by potassium dichromate (K2Cr2O7) and chromium trioxide (CrO3) in acidic media (Jones reagent).
- From alkyl benzenes: Aromatic carboxylic acids can be prepared by vigorous oxidation of alkyl benzenes with chromic acid or acidic or alkaline potassium permanganate. The entire side chain is oxidised to the carboxyl group irrespective of length of the side chain.
- From nitriles and amides: Nitriles are hydrolysed to amides and then to acids in the presence of H+ or OH− as catalyst. Mild reaction conditions are used to stop the reaction at the amide stage.
- From Grignard reagents: Grignard reagents react with carbon dioxide (dry ice) to form salts of carboxylic acids which in turn give corresponding carboxylic acids after acidification with mineral acid.
- From acyl halides and anhydrides: Acid chlorides when hydrolysed with water give carboxylic acids or more readily hydrolysed with aqueous base to give carboxylate ions which on acidification provide corresponding carboxylic acids. Anhydrides on the other hand are hydrolysed to corresponding acid(s) with water.
- From esters: Acidic hydrolysis of esters gives directly carboxylic acids while basic hydrolysis gives carboxylates, which on acidification give corresponding carboxylic acids.
Physical Properties
Aliphatic carboxylic acids upto nine carbon atoms are colourless liquids at room temperature with unpleasant odours. The higher acids are wax like solids and are practically odourless due to their low volatility. Carboxylic acids are higher boiling liquids than aldehydes, ketones and even alcohols of comparable molecular masses. This is due to more extensive association of carboxylic acid molecules through intermolecular hydrogen bonding. The hydrogen bonds are not broken completely even in the vapour phase. In fact, most carboxylic acids exist as dimer in the vapour phase or in the aprotic solvents.
Chemical Reactions
Reaction due to Hydrogen atom of carboxylic acid: This property actually shows acidic character of carboxylic acid acids that is :
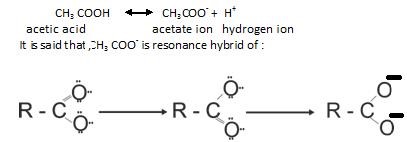
Reactions Involving Cleavage of C–OH Bond:
- Formation of anhydride: Carboxylic acids on heating with mineral acids such as H2SO4 or with P2O5 give corresponding anhydride.
- Esterification: Carboxylic acids are esterified with alcohols or phenols in the presence of a mineral acid such as concentrated H2SO4 or HCl gas as a catalyst.
- Reactions with PCl5, PCl3 and SOCl2: The hydroxyl group of carboxylic acids, behaves like that of alcohols and is easily replaced by chlorine atom on treating with PCl5, PCl3 or SOCl2.
- Reaction with ammonia: Carboxylic acids react with ammonia to give ammonium salt which on further heating at high temperature give amides.
Reactions Involving –COOH Group
- Reduction: Carboxylic acids are reduced to primary alcohols by lithium aluminium hydride or better with diborane. Diborane does not easily reduce functional groups such as ester, nitro, halo, etc. Sodium borohydride does not reduce the carboxyl group.
- Decarboxylation: Carboxylic acids lose carbon dioxide to form hydrocarbons when their sodium salts are heated with sodalime (NaOH and CaO in the ratio of 3 : 1). The reaction is known as decarboxylation.
Substitution Reactions in the Hydrocarbon Part
- Halogenation: Carboxylic acids having an α-hydrogen are halogenated at the α-position on treatment with chlorine or bromine in the presence of small amount of red phosphorus to give α-halocarboxylic acids. The reaction is known as Hell-Volhard-Zelinsky reaction.
- Ring substitution: Aromatic carboxylic acids undergo electrophilic substitution reactions in which the carboxyl group acts as a deactivating and meta-directing They however, do not undergo Friedel-Crafts reaction (because the carboxyl group is deactivating and the catalyst aluminium chloride (Lewis acid) gets bonded to the carboxyl group).
Uses of Uses of Carboxylic Acids
Methanoic acid is used in rubber, textile, dyeing, leather and electroplating industries. Ethanoic acid is used as solvent and as vinegar in food industry. Hexanedioic acid is used in the manufacture of nylon-6, 6. Esters of benzoic acid are used in perfumery. Sodium benzoate is used as a food preservative. Higher fatty acids are used for the manufacture of soaps and detergents.
Advertisement
