The d-block is a group of elements on the periodic table. They include the transition metals. The Group 12 elements are normally called post-transition metals because they are different from the transition metals.
The f-block elements found in the two rows at the bottom of the periodic table, are called inner transition metals and have valence electrons in the f-orbital.
There are mainly four series of the transition metals, 3d series (Sc to Zn), 4d series (Y to Cd), 5d series (Laand Hf to Hg) and 6d series which has Ac and elements from Rf to Cn. The two series of the inner transition metals; 4f (Ce to Lu) and 5f (Th to Lr) are known as lanthanoids and actinoids respectively.
The d–block occupies the large middle section of the periodic table flanked between s– and p– blocks in the periodic table. The d–orbitals of the penultimate energy level of atoms receive electrons giving rise to four rows of the transition metals, i.e., 3d, 4d, 5d and 6d.
In general the electronic configuration of outer orbitals of these elements is (n-1)d 1–10ns 1–2. The (n–1) stands for the inner d orbitals which may have one to ten electrons and the outermost ns orbital may have one or two electrons. However, this generalisation has several exceptions because of very little energy difference between (n-1)d and ns orbitals.
Physical Properties: Nearly all the transition elements display typical metallic properties such as high tensile strength, ductility, malleability, high thermal and electrical conductivity and metallic lustre. With the exceptions of Zn, Cd, Hg and Mn, they have one or more typical metallic structures at normal temperatures. The transition metals (with the exception of Zn, Cd and Hg) are very hard and have low volatility.
Variation in Atomic and Ionic Sizes of Transition Metals: In general, ions of the same charge in a given series show progressive decrease in radius with increasing atomic number. This is because the new electron enters a d orbital each time the nuclear charge increases by unity.
Ionisation Enthalpies: There is an increase in ionisation enthalpy along each series of the transition elements from left to right due to an increase in nuclear charge which accompanies the filling of the inner d orbitals.
Oxidation States: One of the notable features of a transition elements is the great variety of oxidation states these may show in their compounds.
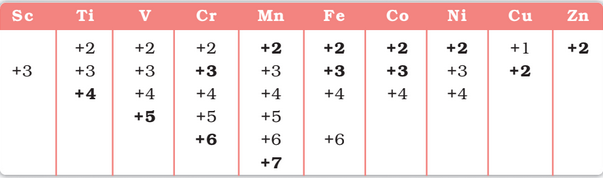
The elements which give the greatest number of oxidation states occur in or near the middle of the series. Manganese, for example, exhibits all the oxidation states from +2 to +7.
The lesser number of oxidation states at the extreme ends stems from either too few electrons to lose or share (Sc, Ti) or too many d electrons (hence fewer orbitals available in which to share electrons with others) for higher valence (Cu, Zn).
Low oxidation states are found when a complex compound has ligands capable of π-acceptor character in addition to the σ-bonding. For example, in Ni(CO)4 and Fe(CO)5, the oxidation state of nickel and iron is zero.
Trends in the M2+/M Standard Electrode Potentials: The stability of the half-filled d sub-shell in Mn2+ and the completely filled d 10 configuration in Zn2+ are related to their EV values, whereas EV for Ni is related to the highest negative ∆hydHV.
Trends in the M3+/M2+ Standard Electrode Potentials: An examination of the EV (M3+/M2+) values (Table 8.2) shows the varying trends. The low value for Sc reflects the stability of Sc3+ which has a noble gas configuration. The highest value for Zn is due to the removal of an electron from the stable d 10 configuration of Zn2+.
Trends in Stability of Higher Oxidation States: the stable halides of the 3d series of transition metals. The highest oxidation numbers are achieved in TiX4 (tetrahalides), VF5 and CrF6. The +7 state for Mn is not represented in simple halides but MnO3F is known, and beyond Mn no metal has a trihalide except FeX3 and CoF3.
Chemical Reactivity and EV Values: Transition metals vary widely in their chemical reactivity. Many of them are sufficiently electropositive to dissolve in mineral acids, although a few are ‘noble’—that is, they are unaffected by single acids.
Magnetic Properties: The d-block element in the periodic table will show the magnetic property as their (n-1) d orbital owns the unpaired electrons. The higher the number of the unpaired electron in (n-1) element electronic configuration, they will tend to achieve the maximum magnetic behaviour.
Formation of Coloured Ions: During the d – d transition process the electrons absorb certain energy from the radiation and emit the remainder of energy as coloured light. The colour of ion is complementary of colour absorbed by it. Hence coloured ion is formed due to d – d transition which falls in the visible region for all transition elements.
Formation of Complex Compounds: They are of proper energy to believe lone pair and unshared pair of electrons from the ligands. Hence conversion elements form complexes simply. Most subsist in numerous oxidation states and form many multifaceted ions and colored compounds. The partly filled subshells of d-block elements incorporate (n-1) d subshell.
Catalytic Properties: The transition metals and their compounds are known for their catalytic activity. This activity is ascribed to their ability to adopt multiple oxidation states and to form complexes. Vanadium(V) oxide (in Contact Process), finely divided iron (in Haber’s Process), and nickel (in Catalytic Hydrogenation) are some of the examples.
Formation of Interstitial Compounds: Interstitial compounds are those which are formed when small atoms like H, C or N are trapped inside the crystal lattices of metals. They are usually non stoichiometric and are neither typically ionic nor covalent, for example, TiC, Mn4N, Fe3H, VH0 .56 and TiH1.7, etc. The principal physical and chemical characteristics of these compounds are as follows:
(i) They have high melting points, higher than those of pure metals.
(ii) They are very hard, some borides approach diamond in hardness.
(iii) They retain metallic conductivity.
(iv) They are chemically inert.
Alloy Formation: An alloy is a blend of metals prepared by mixing the components. Alloys may be homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms of the other. Such alloys are formed by atoms with metallic radii that are within about 15 percent of each other. Because of similar radii and other characteristics of transition metals, alloys are readily formed by these metals.
These oxides are generally formed by the reaction of metals with oxygen at high temperatures. All the metals except scandium form MO oxides which are ionic. The highest oxidation number in the oxides, coincides with the group number and is attained in Sc2O3 to Mn2O7. Beyond group 7, no higher oxides of iron above Fe2O3 are known. Besides the oxides, the oxocations stabilise VV as VO2+, VIV as VO2+ and Ti IV as TiO2+.
Potassium dichromate (K2Cr2O7): Potassium dichromate is a very important chemical used in leather industry and as an oxidant for preparation of many azo compounds. Dichromates are generally prepared from chromate, which in turn are obtained by the fusion of chromite ore (FeCr2O4) with sodium or potassium carbonate in free access of air.
Potassium permanganate (KMnO4): Potassium permanganate is prepared by fusion of MnO2 with an alkali metal hydroxide and an oxidising agent like KNO3. This produces the dark green K2MnO4 which disproportionates in a neutral or acidic solution to give permanganate.
The f-block consists of the two series, lanthanoids (the fourteen elements following lanthanum) and actinoids (the fourteen elements following actinium). Because lanthanum closely resembles the lanthanoids, it is usually included in any discussion of the lanthanoids for which the general symbol Ln is often used.
The names, symbols, electronic configurations of atomic and some ionic states and atomic and ionic radii of lanthanum and lanthanoids (for which the general symbol Ln is used) are
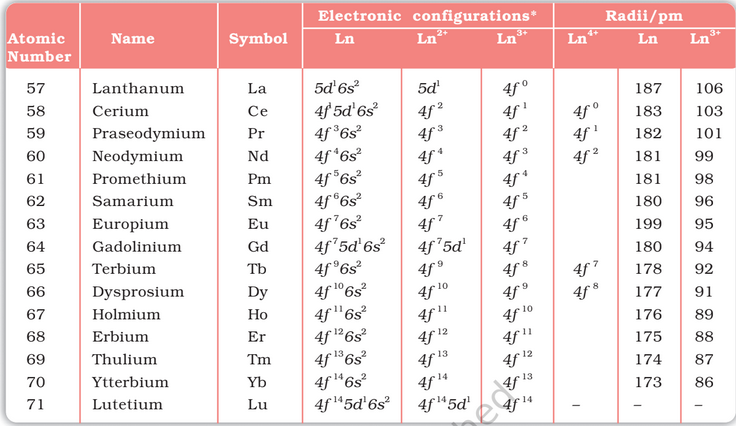
General Characteristics: All the lanthanoids are silvery white soft metals and tarnish rapidly in air. The hardness increases with increasing atomic number, samarium being steel hard. Their melting points range between 1000 to 1200 K but samarium melts at 1623 K. They have typical metallic structure and are good conductors of heat and electricity. Density and other properties change smoothly except for Eu and Yb and occasionally for Sm and Tm.
The actinoids include the fourteen elements from Th to Lr. The names, symbols and some properties of these elements are
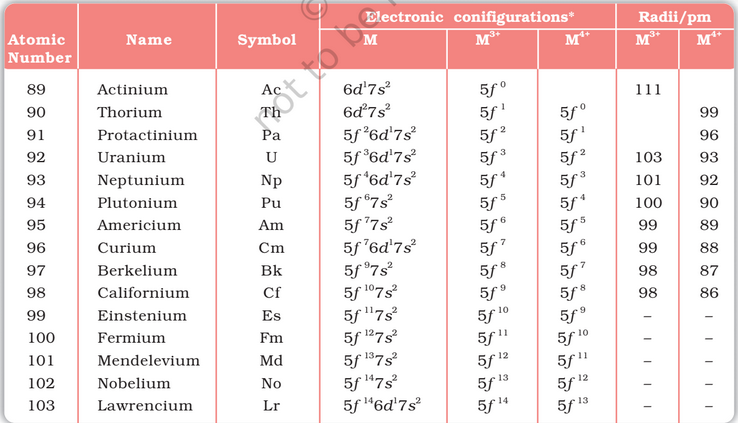
Electronic Configurations: All the actinoids are believed to have the electronic configuration of 7s2 and variable occupancy of the 5f and 6d subshells. The fourteen electrons are formally added to 5f, though not in thorium (Z = 90) but from Pa onwards the 5f orbitals are complete at element 103. The irregularities in the electronic configurations of the actinoids, like those in the lanthanoids are related to the stabilities of the f0, f7 and f14 occupancies of the 5f orbitals. Thus, the configurations of Am and Cm are [Rn] 5f 7 7s2 and [Rn] 5f 76d 1 7s2.
Ionic Sizes: The general trend in lanthanoids is observable in the actinoids as well. There is a gradual decrease in the size of atoms or M3+ ions across the series. This may be referred to as the actinoid contraction (like lanthanoid contraction). The contraction is, however, greater from element to element in this series resulting from poor shielding by 5f electrons.
Oxidation States: There is a greater range of oxidation states, which is in part attributed to the fact that the 5f, 6d and 7s levels are of comparable energies.
General Characteristics and Comparison with Lanthanoids:
| Article and Schedule Quiz | Start Test! |