1
KMnO4 reacts with oxalic acid according to the equation given below. Here 20 mL of 0.1 M KMnO4 is equivalent to how many mL and molarity of H2C2O4?
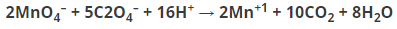
Answer: 50 mL of 0.1 M H2C2O4
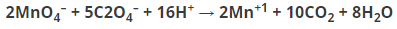
Answer: 50 mL of 0.1 M H2C2O4
Answer: CuI2 is formed
KMnO4 + H2C2O4 → 2C02 + Mn2+
Answer: 4/5
Answer: 98 g
Answer: 120 ml
Answer: 10.44 g
Answer: 2.5 M
Answer: 16.66 mole
How many moles of KMnO4 are required for complete oxidation of 1.25 mol Cu2S?
Cu2S → Cu+2 + SO2
Answer: 2 moles
Answer: 45