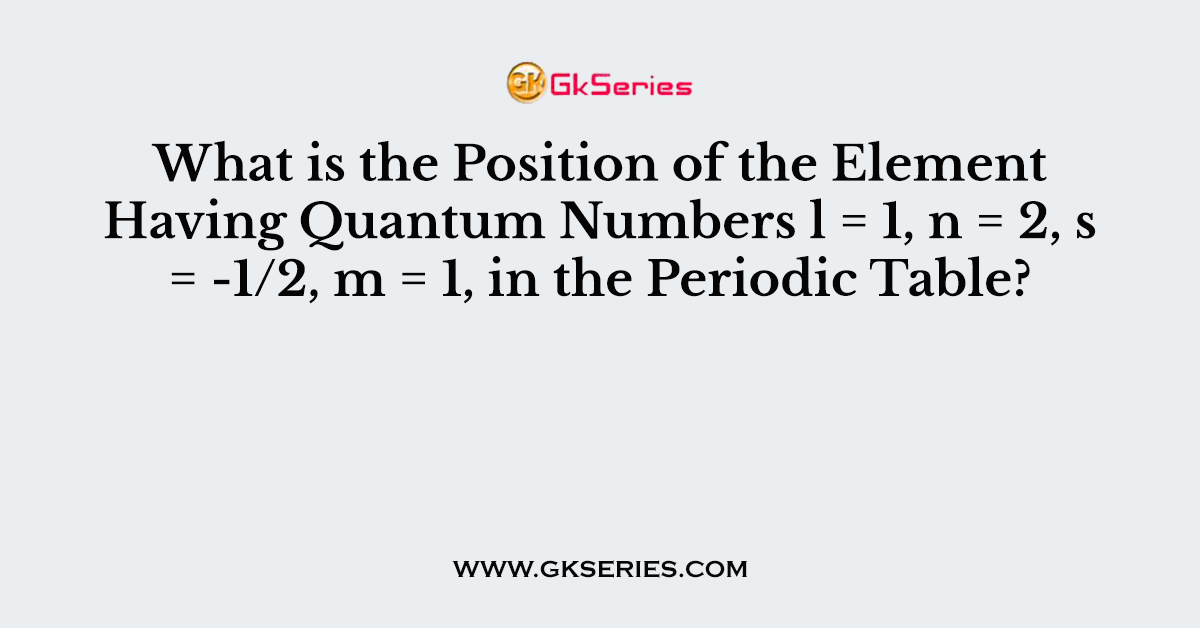
Q. What is the Position of the Element Having Quantum Numbers l = 1, n = 2, s = -1/2, m = 1, in the Periodic Table?
A. Group 0, period II.
B. Group VIIA, period II.
C. Group 0, period III.
D. Group VIIA, period III.
Answer: Group 0, period II.

A. Group 0, period II.
B. Group VIIA, period II.
C. Group 0, period III.
D. Group VIIA, period III.
Answer: Group 0, period II.