Answer:NH3
Answer:unchanged
| Article and Schedule Quiz | Start Test! |
![]()
Answer:64 times
Answer:4
at the same temperature, what will be the equilibrium constant for the following reaction?
Answer:6 x 10-2
Answer:BaCl2
If the equilibrium constant for the following reactions are given
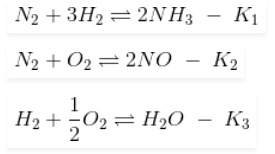
Answer:K2K33/K1
Answer:0.013%
Answer:HCl
Answer:12.65
Answer:A, D
Answer:A, C, D
Answer:The equilibrium will remain unaffected in all the three cases.
1/2 H2 (g) +1/2 I2 (g) ⇌ HI (g)
What would be the equilibrium constant Kc for the reaction 2HI (g) ⇌ H2 (g) + I2 (g)
Answer:0.04
Answer:Water < acetone < ether
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
Which of the following is correct, if the total pressure at which the equilibrium is established, is increased without changing the temperature?
Answer:K will remain the same
Answer:∆Gᶱ= 0
Answer: 7.0
Answer:3.4
Answer:Aqueous ammonia