1
What is the concentration of carbon dioxide in the atmosphere?
Answer: 3.5 x 106 ppm
Answer: 3.5 x 106 ppm
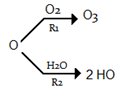
Answer: R1 << R2
Answer: Alkanes, the hydroxyl radical abstracts a hydrogen atom and forms a carbanion
Answer: exothermic
Answer: It cannot be a negative number
Answer: ∆Hrxn = Σ∆Hf (product) – Σ∆Hf (reactant)
H2 (g) + 1/2 O2(g) → H2O(g), ∆H=-241.8 KJ
C2H2 (g) + 5/2 O2(g) → CO2(g) + H2O(g), ∆H=-1300 KJ
Answer: 1/5.37
Answer: 39.4 KJ
Answer: ∆Gtransition of C (diamond) → C (graphite) is -ve
Answer: Smouldering